Introduction
We’re going back to the basics of the physics of electricity and magnetism. The overall goal of the combined blogs is to help you solidify your model of charge flow in conductors.
The last blog of the previous blog mini-series covered the concept of energy and reminded readers that energy doesn’t exist. Energy, in its various forms, is simply a calculated mathematical invention based on carefully chosen object properties and constants of proportionality.
This blog series will remind readers about the ideas of electric force and electric fields.
The universe is a complicated place that’s been around for 13.8 billion years. Scientists have only begun to unlock the more interesting secrets in the last 200-300 years. That means there’s still plenty to discover and understand. In the first blog in our Back-to-Basics series, we took a look at the historical origins of Ohm’s law and Joule’s power law. In the second blog, we investigated the concept of Energy.
Back-to-Basics Series 1 Links: The Problem with the Drude Model
- Drude Was a Flimflam Man
- If a Spark Jumps in a Lab and the Scientist Doesn’t Report It, Does it Really Make a Shock?
- Repeal Ohm’s Law!
- Fight the Power and Kirchoff Flips the Switch on Ohm’s Law.
Back-to-Basics Series 2 Links: What is Energy?
- Repeat After Me: “That Doesn’t Make Any Sense!”
- The Little Engine that Could Change the World
- Enough Already! What is Energy?
Now we pivot to discuss the electric field…
What is Electric Charge?
In the last blog series, we learned that every object has unique object properties. The properties are given by nature, then identified and labeled by scientists.
This abbreviated list of object properties was taken from Wikipedia.org and turned into a word cloud.
Electric charge is one property of matter that affects an object’s behavior during interactions with other objects. The theory of Quantum Electrodynamics (QED) breaks protons’ and electrons’ behavior down a bit further. But the time spent discussing quantum theory is significant, and the increase in an engineer’s understanding of circuit behavior based on that theory is negligible, so we’ll skip it for now[1]. For our purposes, it is sufficient to say that a property called charge exists. Particles that have a non-zero charge will interact with other particles with a non-zero charge in measurable and predictable ways.
The property of charge originates at the sub-atomic level with the proton and electron. As far as any experiments have ever shown, protons and electrons have exactly the same magnitude of charge. However, they do have a distinguishing attribute we describe mathematically with plus and minus signs. Charge magnitude comes in values that are always integer multiples of 1.6 x 10-19 C. The rules of the universe require that identically signed charges repel and oppositely signed charges attract. We don’t necessarily know why they behave this way – we’ve just observed it and then created a mathematical model that fits the behavior.
For mathematical convenience, protons are defined to possess a positive charge p = +1.6 x 10-19 C and electrons are defined to possess a negative charge[2] e– = –1.6 x 10-19 C.
Inertia and Forces
The rules of the universe also require that all objects move with constant velocity (straight lines at a constant speed.) The only way to change the velocity (speed and/or direction) of an object is through interaction with another object.
Electric Force and Coulomb’s Law
One such interaction is the electric force.
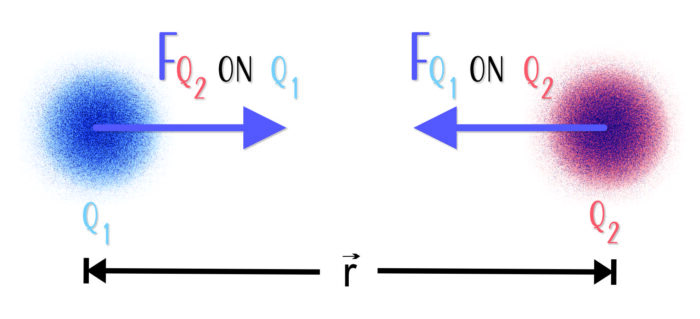
Two charges, Q1 and Q2, separated by a distance r, experience identical forces of attraction.
The magnitude of the force between any two point charges is proportional to the product of the particles’ electric charge q and inversely proportional to the square of the distance between the particles →r. Charge comes in multiples of 1.6 x 10-19 C and is carried on protons and electrons[3]. The direction of force lies along a straight line r that joins the two objects.
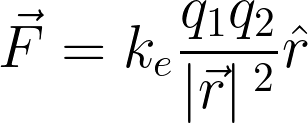
The constant of proportionality is
The equation is known as Coulomb’s law. Every experiment performed has shown that Coulomb’s law is always true. We know that two charges will interact and experience a force in a direction along a line that joins the two points.
Three or More Charged Objects
By the time you investigate three charges, you usually have to keep track of the result of forces in two dimensions.
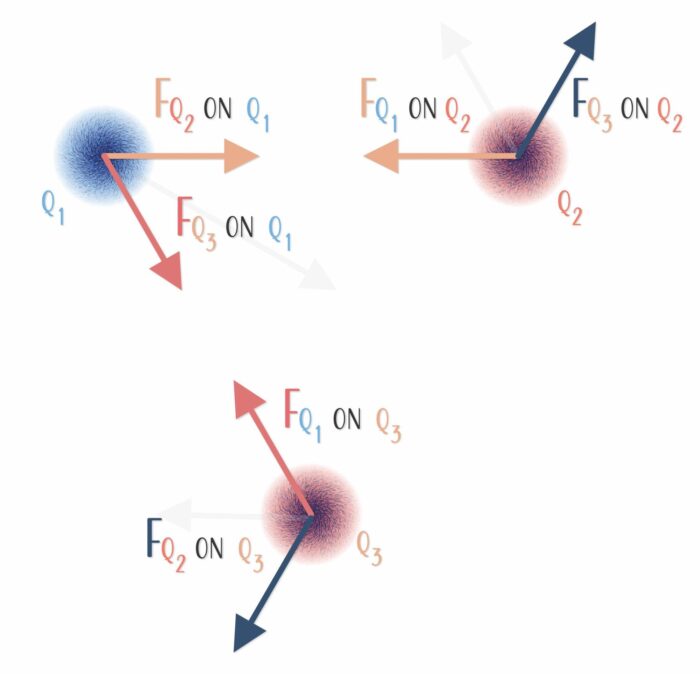
And with four charges comes the likelihood of keeping track of forces in three dimensions.
The Superposition Principle
Determining the effects of multiple charges is relatively simple. The resulting force is the vector sum of all the individual forces on a given charge. ΣF = F1 + F2 + … + Fn. Superposition in this context simply means that each interaction’s contribution is independent of all other interactions’ contributions.
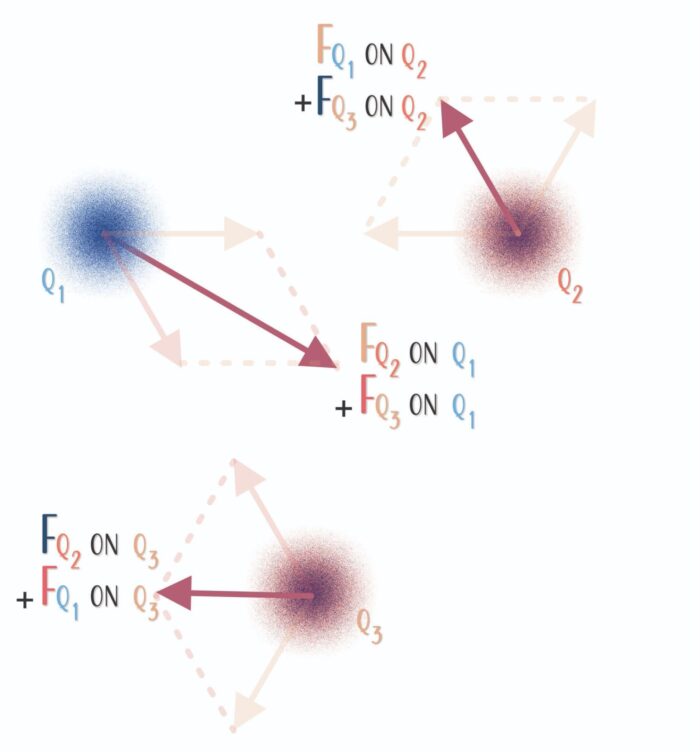
The net force on each charge is the sum of all the electric forces acting on that charge.
That might seem like a logistic nightmare because it is rare to find two point-charges alone in nature. Usually, where you find two charges, you’ll find several hundred thousand billion billion more within a centimeter radius. But do you even need to consider every charge in a centimeter radius? How about a micrometer radius? Or a nanometer radius?
Situations involving multiple charges get complicated quickly.
In the next blog, we will build the ideas of fields and we will ask what, if anything, happens in the space that lies off-axis or on the far side of the charges? In a future blog, we will develop an intuition for the separation distances at which the charges no longer interact in a meaningful manner.